No. 68: High Steam Quality
Do you want this article in PDF format? Download it here:
Download a PDFHIGH STEAM QUALITY IS ESSENTIAL FOR THE STEAM SYSTEM
UNDERSTANDING AND TESTING
1. WHAT IS STEAM QUALITY?
Steam quality is the proportion of saturated steam (vapor) in a saturated condensate (liquid)/steam (vapor) mixture. A steam quality of 0 indicates 100% liquid (condensate), while a steam quality of 100 indicates 100% steam. One pound of steam with 95% steam and 5% of liquid entrainment has a steam quality of 0.95.
The measurements needed to obtain a steam quality measurement are temperature, pressure, and entrained liquid content. A high percentage (88% or more) of industrial steam systems use saturated steam for process applications. Saturated steam (meaning steam that is saturated with energy) is completely gaseous and contains no liquid.
The boiler operation uses chemical energy from a fuel source to deliver energy to the boiler water. Inside the boiler, liquid gains energy from the combustion process and changes state into saturated steam. Water enters the boiler at point A, and the water gains sensible energy (hf) to point B. The change of state is referenced as point B in Figure 1. As the saturated steam acquires more energy from the boiler combustion process, the steam achieves a high quality (moving left to right), as represented by points B to C. The increase in energy gained by the steam from points C to D goes toward the superheat of the vapor.
Figure 1. Relationship of Enthalpy and Temperature.
A directly proportional relationship exists between temperature and pressure in saturated steam. This means that as the temperature increases, so does the pressure. As illustrated by the lines of constant pressure in Figure 1, more sensible energy (hf) is needed for water to transition from point A to point B and become a vapor.
When steam enters the process, the energy level goes from right to left as the process absorbs the energy from the steam.
1.1. Why Steam Quality Is Important
Today’s manufacturing techniques of heat transfer and control and related standards are all dedicated to improving and providing the highest-quality product to the marketplace. To attain the highest quality, each manufactured component of the final product is inspected repeatedly and measured. This ensures that it meets the manufacturer’s and consumer’s expectations.
Steam plays a vital role in producing the final product; therefore, steam quality should be one of the main measurable points in creating a product in today’s manufacturing facilities. All heat transfer components (e.g., shell/tube, plate/frame, plate/coil, and tracing) base performance calculations on 100% steam quality, unless the manufacturer is informed by the end user that the steam quality is lower than 100%.
Unfortunately, steam quality is typically not monitored closely and is often assumed to be 100%. Therefore, issues that arise from poor steam quality are blamed on some other item in the system. Based on field documentation by Inveno Engineering Steam Team, a high percentage of steam systems are operating below acceptable steam quality levels.
1.2. What Are the Effects of Steam Quality?
Low steam quality affects steam system operations in many ways. Here are four:
- Reduced heat transfer efficiency: The major problem with low steam quality is the effect on the heat transfer equipment and process. In some cases, low steam quality can reduce heat transfer efficiency by more than 65%. The liquid entrained in the steam has sensible energy (estimated at 16%, but varies with pressure), which has a significantly lower amount of energy than the steam vapor’s latent energy (94%). Therefore, less usable energy is being delivered to the steam process equipment.
Also, the additional liquid (low steam quality) collects on the wet surface of the heat exchanger, causing an additional buildup of a liquid, which reduces the ability of the steam’s latent energy to be transferred to the product.
- Premature valve failure: Liquid passing through steam control valves will erode the internals of the valves, causing premature failure.
- Internal turbine component failures: Liquid introduced with the steam in a saturated turbine operation will reduce the life expectancy of the internal components.
- Water hammer: Steam systems are usually not designed to accommodate the additional liquid in steam. Additional liquid creates the chance for water hammer to occur. Water hammer poses a safety risk and may cause premature failure in the steam system.
2. HOW TO VISUALLY MEASURE STEAM QUALITY
Figure 2. Acceptable Steam Quality/
A true measurement of steam quality can be obtained from the use of a throttling calorimeter and Ganapathy’s steam plant calculations. Unfortunately, most industrial plants do not have the luxury or capability of doing the steam quality testing.
Another way to measure steam quality is by relying on the basics of steam. Saturated steam is a dry invisible gas and only becomes visible with entrained air or liquid. Therefore, opening a steam valve and allowing steam to be released into the atmosphere provides an estimate of the steam quality in the system. This is a steam quality test that all plants can conduct on a routine basis.
2.1. Examples
Figure 2 indicates an acceptable steam quality. The discharge from the valve through the tube is almost invisible.
Figure 3. Unacceptable Steam Quality.
Figure 3 shows the discharge from the valve off the steam line to be very visible, with liquid being discharged with the steam vapor.
The steam quality is not acceptable for the process.
Figure 4 shows the discharge from the valve off the steam line to be very visible, with liquid being discharged with the steam vapor.
Figure 4. Unacceptable Steam Quality.
The steam quality is not acceptable for the process.
3. STEAM CALORIMETER
Figure 5. Steam Calorimeter Stand.
The measuring device used to determine the moisture content of steam is called a steam calorimeter. However, it really does not measure the heat in the steam, so the name does not reflect the actual function of the measuring device. The first known name used was the “barrel calorimeter,” but the likelihood of error was so great that the device was totally abandoned. Modern calorimeters are generally either throttling or separator measuring devices.
All steam quality measuring devices use the same principle, which will be described in the pressure-reducing section of this technical paper.
3.1. Throttling Calorimeter
Figure 6. Throttling Calorimeter.
Figure 6 shows a typical form of throttling steam calorimeter. Steam is flowing from a vertical main steam line through the sampling nipple. The steam flows around the first measuring thermometer cup, then passes through a ⅛” orifice in a disk between two flanges, goes around the second measuring thermometer cup, and is released to the atmosphere.
The instrument and all pipes and fittings leading to it should be thoroughly insulated to diminish the energy loss that can affect the measurement.
The small orifice can have issues with corrosion material flowing through the measuring device. Therefore, proper steam filtration needs to be part of the measuring system.
The discharge steam piping needs to be short to prevent any backpressure below the disk area, which could cause measurement errors.
3.2. Compact Throttling Calorimeter
Figure 7. Compact Throttling Calorimeter.
There are many forms of throttling calorimeter, all of which work upon the same principle. A compact throttling calorimeter consists of two concentric metal cylinders connected to a cap containing a thermometer well. The steam pressure is measured by a gauge placed in the steam supply pipe or other convenient location.
Steam passes through the orifice (note A) and expands to atmospheric pressure. The steam temperature is measured by a thermometer placed in the cup (note C).
To prevent radiation losses, the annular space between the two cylinders is used as an insulating jacket. Steam is supplied to this space through the hole (B).
3.3. Separating Calorimeter
Figure 8. Separating Calorimeter.
The separating calorimeter mechanically separates the entrained water from the steam and collects it in a reservoir, where its amount is either indicated by a gauge glass or where it is drained off and weighed. Figure 8 shows a calorimeter of this type.
The steam passes out of the calorimeter through an orifice of known size so that its total amount can be calculated or can be weighed. A gauge is ordinarily provided with this type of calorimeter, which shows the pressure in its inner chamber and the flow of steam for a given period, this latter scale being graduated by trial.
The instrument, like a throttling calorimeter, should be well insulated to prevent losses from radiation.
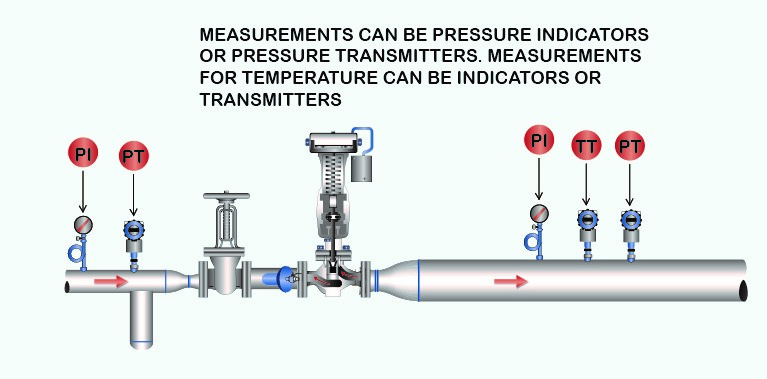
4. STEAM PRESSURE-REDUCING STATION: TESTING STEAM QUALITY
Figure 9: Pressure Reducing Station.
A steam pressure-reducing valve station will work the same as a throttling calorimeter. In a typical installation of a steam pressure-reducing station, adding pressure measurements upstream and downstream with the addition of a temperature measurement downstream provides the continuous online “steam calorimeter.”
When steam passes through an orifice (valve internals) from a high steam pressure to a lower steam pressure, as is the case with the throttling calorimeter, no external work has to be done in overcoming a resistance. Hence, if there is no loss from radiation, the quantity of heat in the steam will be exactly the same after passing the orifice (valve internals) as before passing or the valve inlet Btu quantity.
Example:
- Inlet to pressure-reducing valve: 150 psig
- Total energy at 150 psig: 1,196 Btu/lb.
- Outlet of pressure-reducing valve: 15 psig
- Outlet temperature (saturated conditions) 15 psig: 7°F
- Total energy at 15 psig: 1,164 Btu/lb.
- Difference in Btu/lb. from high pressure to low pressure: 32 Btu/lb.
Figure 11: Superheat Results.
Figure 10: Example of Btu Differences.
A difference of 32 Btu/lb. exists after the pressure-reducing station at the lower steam pressure (15 psig) because no external work was accomplished. The 32 Btu/lb. will create the effect of superheat. Assuming the specific heat of superheated steam to be 0.52, each pound passing through will be superheated 32/.52 = 61.5°. Therefore, the downstream temperature, if 100% steam quality exists, would be 311.2°F.
For the example, if the steam had contained 1% of moisture, it would have contained fewer heat units per pound than if it were dry steam. Since the latent heat of steam at 150 psig is 857.4 Btu/lb., it follows that the 1% of moisture would have required 8.5 Btu/lb. to evaporate it, leaving only 32 – 8.5 = 23.5 Btu/lb. available for superheating; hence, the superheat would be 23.5⁄0.52 = 45.1°F, compared to 61.5°F for dry steam.
The degree of superheat for other percentages of moisture may be determined. The action of the throttling calorimeter is based upon the foregoing facts, as shown below.
- H = total heat of one pound of steam (inlet steam pressure) to the valve station
- L = latent heat of steam at the inlet to the valve station
- h = total heat of steam at the reduced steam pressure or outlet of the valve station
- t1 = temperature of saturated steam at the reduced steam pressure or outlet of the valve station
- t2 = temperature of saturated steam at the reduced steam pressure or outlet of the valve station
- 52 = specific heat of saturated steam at the outlet steam pressure of the outlet of the valve station
- x = proportion by weight of moisture in steam
The difference in Btu/lb. of steam at the inlet valve station pressure and after passing the orifice (valve internals) is the heat available for evaporating the moisture content and superheating the steam. Therefore,
H – h = xL + 0.52 (t2 –t1)
or
x = H – h – 0.52 (t2 –t1)
L
Almost invariably, the lower pressure is taken as that of the atmosphere. Under such conditions, h = 1,163.9 and t1 = 249.5°F.
A slight error may arise from the value used as the specific heat of superheated steam at the example lower steam pressure of 15 psig: 0.52. However, any error resulting from its use will be negligible.