No. 35 – Combustion Testing Sheets
Do you want this article in PDF format? Download it here:
Download a PDFCombustion Testing Process and Documentation
The combustion process in the steam boiler transfers the chemical energy to thermal energy, which in turn is absorbed by the water in the boiler. To measure combustion process and efficiency, the plant must have equipment or have an outside firm analyze the flue gases from the combustion process. Combustion refers to the rapid chemical union of oxygen with fuel.
With today’s high energy costs and the need to reduce emissions, it is necessary to obtain data from the steam boiler at different firing rates during the combustion testing process. (See attached combustion testing sheet.)
Outside Combustion Testing Contractor
If anoutside firm is used for the combustion testing procedure, the plant needs to ensure the company and individual are qualified to conduct the combustion test. A number of items should be provided by the outside firm:
- Insurance documentation to meet the plant requirements.
- Standard operating procedures (SOPs) on the combustion testing process
- The SOP should be a step by step documented procedure. A typical SOP is five to eight pages in length
- Validation and calibration of the combustion analyzer and any other test instruments used for the combustion testing
- A sample of the report that will be delivered after the testing is completed
- A sample of the combustion testing data sheet that will be used during the combustion testing procedure (see a sample combustion testing data sheet at the end of this Best Practice)
Figure 1: Unacceptable Combustion Data Reporting
Combustion Testing Data Sheet
It is extremely important to have all the data taken during combustion testing to establish the boiler benchmarks for comparison against the original boiler manufacturer’s benchmarks. This Best Practice includes a sample combustion testing data sheet for review.
Do not let the combustion testing person(s) provide the plant with only the printouts off a combustion analyzer. That is only a very small percentage of the required information to properly manage the boiler operation and energy.
As viewed in the sample combustion testing data sheet, items such as burner ring pressure, windbox pressure, flue gas temperatures, etc. are a small number of the required items. All data has to be taken on ten different points of the firing rate of the boiler.
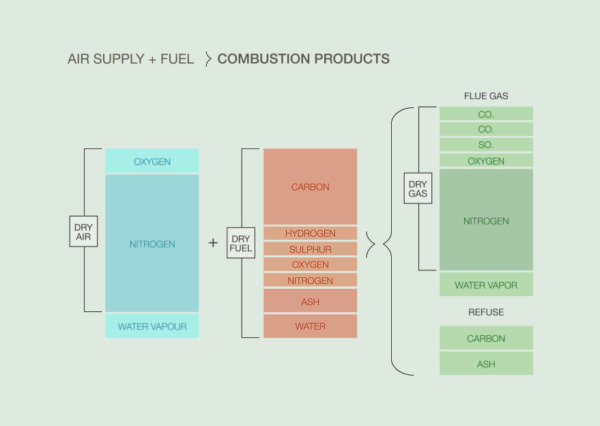
Figure 2: Combustion Process
Combustion Overview
The perfect combustion of fuel would result in:
- Carbon dioxide
- Nitrogen
- Water vapor
- Sulphur dioxide
Figure 3: The Fire Triangle
The oxygen required to burn the fuel is obtained from the air. Air is a mechanical mixture containing by weight 21% oxygen, 78% nitrogen, and 1% other gases. Only oxygen is used in combustion. Nitrogen is an inert gas that has no chemical effect upon combustion.
The chemical combination obtained during combustion results in the liberation of heat energy. Actually, what happens is a rearrangement of the atoms of the chemical elements into new combinations of molecules. In other words, when the fuel temperature (in the presence of oxygen) is increased to the ignition point, a chemical reaction occurs. The fuel begins to separate and unite with specific amounts of oxygen to form an entirely new substance. Heat energy is given off in the process.
Perfect combustion is the objective. However, this has been impossible to achieve as yet in either a boiler or the cylinders of an internal-combustion engine. Theoretically, it is simple. It consists of bringing each particle of the fuel (heated to its ignition temperature) into contact with the correct amount of oxygen.
The following factors are involved:
- Sufficient oxygen must be supplied.
- The oxygen and fuel particles must be thoroughly mixed.
- Temperatures must be high enough to maintain combustion.
- Enough time must be allowed to permit completion of the process.
Complete combustion can be achieved. This is accomplished by more oxygen being supplied to the process than would be required if perfect combustion were possible. The result is that some of the excess oxygen or excess air appears in the combustion gases.